Novel Tetrapyrrolic Macrocycles as PDT and Theranostic Agents for Cancer
Pinho e Melo’s and Filomena Botelho’s research groups, have previously worked on a joint research project focused on the development of “Novel Fused Ring Tetrapyrrolic Macrocycles as Highly Efficient Theranostic Agents for Cancer “, a FCT financed project (PTDC/QEQ-MED/0262/2014; PI: Pinho e Melo).
The porphyrin-type photosensitizers developed by our group, a new type of stable 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins obtained via an unprecedented [8π+2π] cycloaddition of diazafulvenium methides with porphyrins, proved to be very active photodynamic agents against several types of cancer. Furthermore, the in vivo evaluation of the more promising ring-fused diphenylchlorins, using an OE19 tumor/chick embryo chorioallantoic membrane (CAM) model, showed a tumor weight regression of 33% with a single-PDT treatment with the photosensitizer dose as low as 37 ng/embryo [Eur. J. Med. Chem. 2015, 103, 374; Eur. J. Med. Chem. 2018, 146, 395; ACS Omega 2019, 4, 17244; Front. Chem. 2022, 10:873245].
The study of fluorinated ring-fused chlorins as PDT agents against melanoma and esophagus cancer was also carried out. It was observed that the presence of short-chain PEG groups together with the diol moiety allows the modulation of photophysical characteristics (decreases the fluorescence quantum yield and increases singlet oxygen formation quantum yield) and improves cellular uptake leading to very active photosensitizers [RSC Medicinal Chemistry 2021, 12, 615-627].
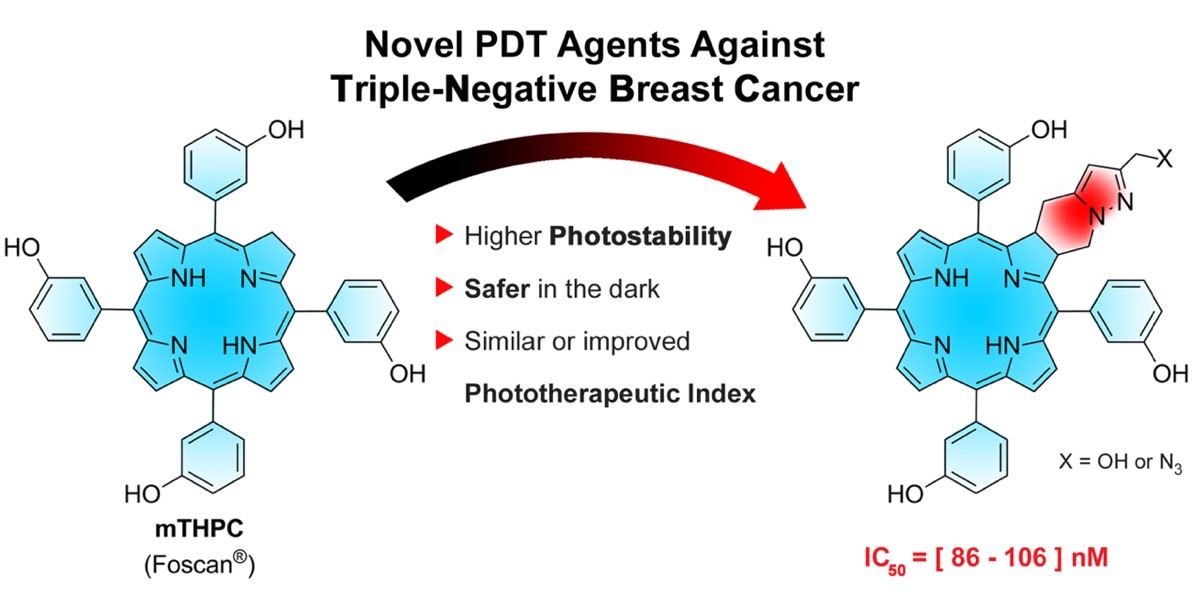
Foscan-derived ring-fused chlorins for photodynamic therapy of cancer have also been developed. These chlorins have photochemical properties similar to Foscan®, a clinically‑approved chlorin, but are much more photostable, safer in the dark and with similar or improved Phototherapeutic Index [Bioorg. Med. Chem. 2023, 93, 117443].
Foscan-Derived Ring-fused Chlorins for Photodynamic Therapy of Cancer
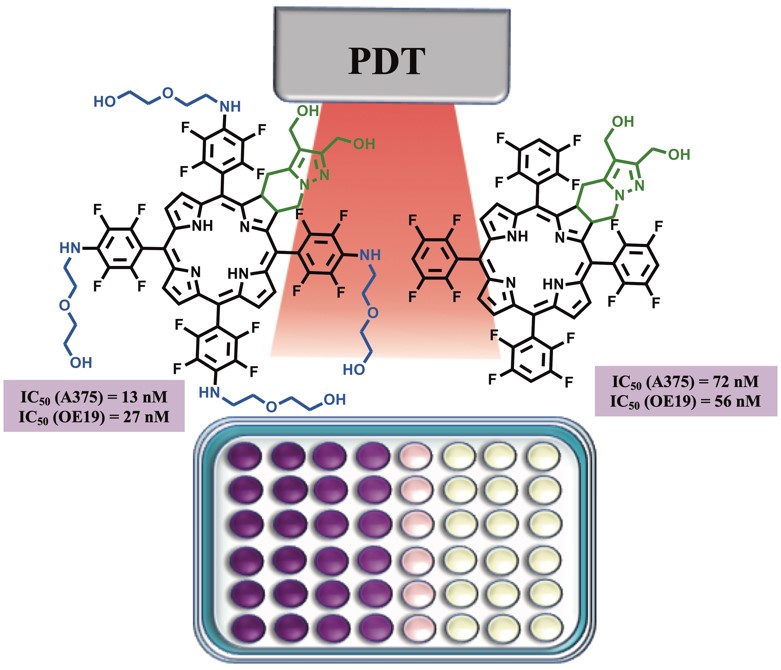
Theranostics, which is the integration of imaging and therapy in a single system, allows simultaneous image-guided therapy and follow-up of the treatment outcome at an early stage, making it possible to increase treatment efficiency. We have demonstrated that the incorporation of the platinum (II) into the structure of 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins leads to molecules with features of theranostic agents. In fact, platinum (II) derivatives of 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins prove to be very promising theranostic agents acting as near-infrared luminescence probes as well as photosensitizers for PDT. Moreover, the remarkable photophysical features of these compounds, including oxygen-dependent room temperature phosphorescence and potential in ratiometric emission measurements, make them excellent compounds to be used as probes for molecular oxygen in biological systems [ACS Med. Chem. Lett. 2017, 8, 310−315]

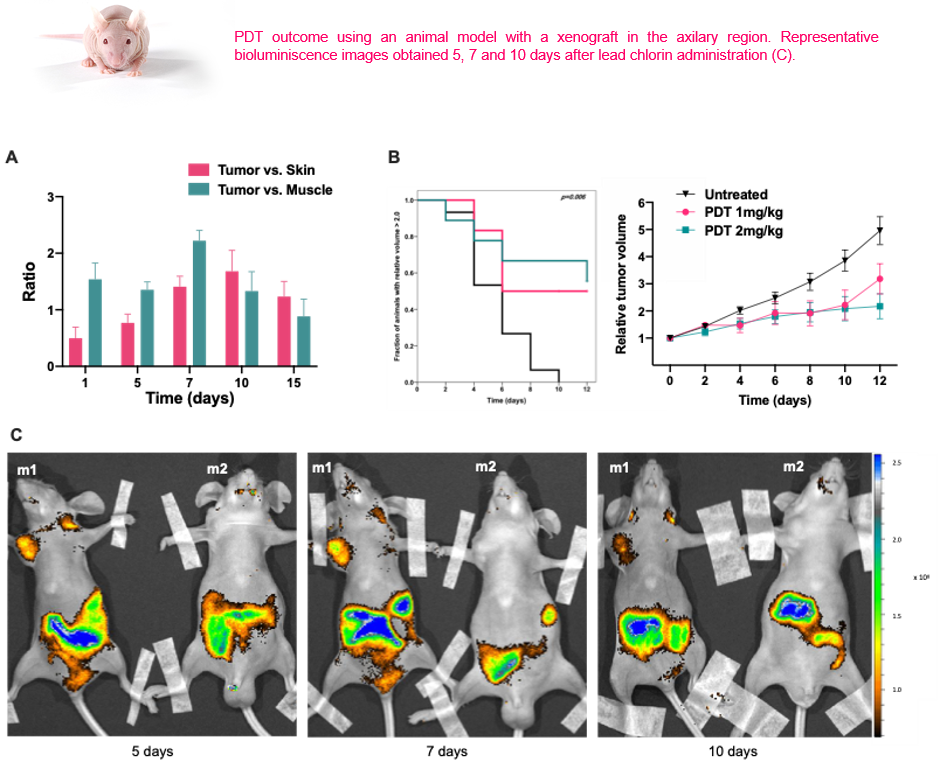
In vivo studies using a A375 melanoma mouse model, proved the extraordinary properties of this chlorin as a luminescent probe and the ability to impair tumor growth, making image guided treatment and follow up a possibility [Eur. J. Med. Chem. 2020, 200, 112468].
Further studies are being carried out namely the optimization of the lead compound and its pre-clinical validation aiming at the development of new tools for personalized cancer treatment. In this context, research has been carried in the framework of a financed project focused on the Validation of Novel Cancer Theranostic Agents [Project's Acronym: TheraBullet; Proof of Concept Project (PdC): CENTRO-01-0145-FEDER-180078; PI: Teresa Pinho e Melo; Co-PI: Marta Pineiro].
Novel PolyPyrrole Scaffolds for Therapy and Imaging of Lung Cancer
The focus of this ongoing project is to explore innovative chemistry for the synthesis of new pyrrolic compounds to be used on PDT and imaging of lung cancer. The aim is to obtain new structures with strategic functional groups, namely oxime or hydrazone functionalities, to tune the solubility of these heterocycles in biological media as well as to modulate the photophysical properties in view of several biological applications [FCT-project: PTDC/QUI-QOR/0103/2021; PI: Teresa Pinho e Melo; Co-PI: Marta Pineiro].
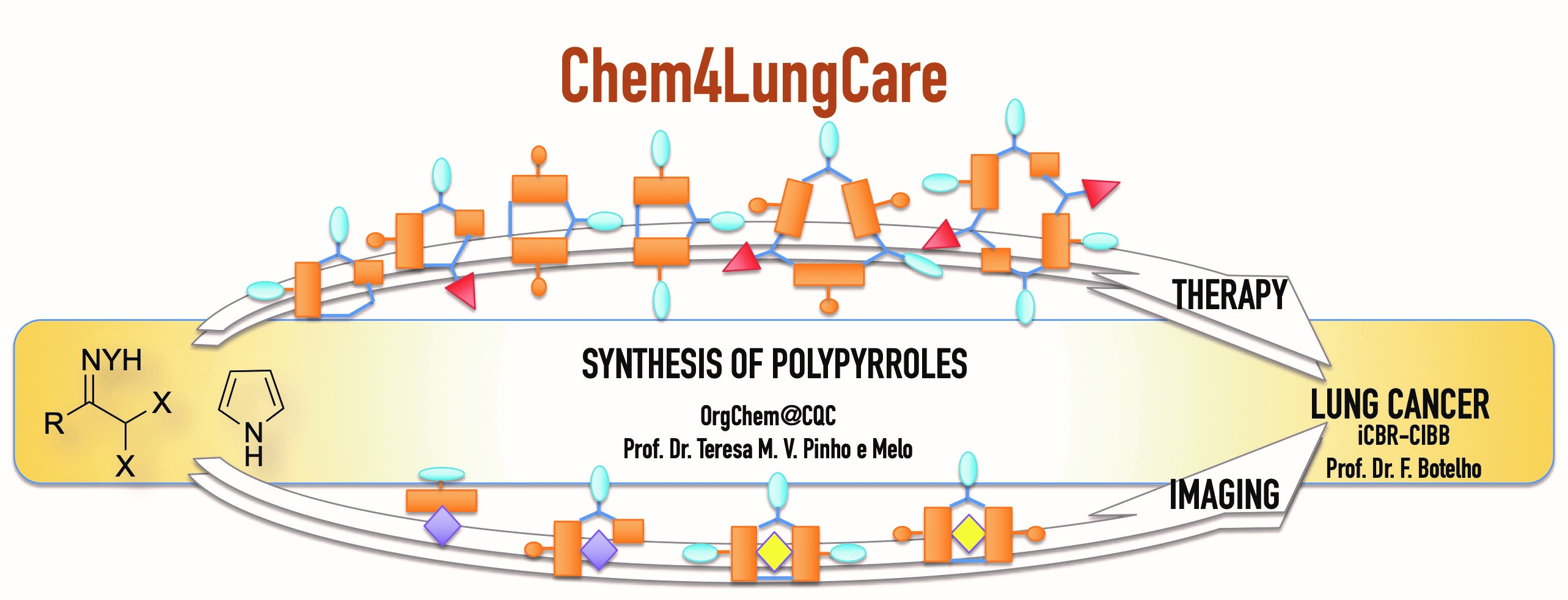
The chemistry of nitrosoalkenes and azoalkenes is one of our research topics, applied to the alkylation of five-membered heterocycles and on the development of new routes to heterocycles (Chem. Rev. 2018, 118, 11324). The assembly of heterocyclic systems using the chemistry of these heterodienes included one-pot synthetic approaches to bis(heterocycle)methanes bearing an oxime or hydrazone functionality. Dehydrohalogenation of α,α-dihalo-oximes and α,α-dihalo-hydrazones in presence of pyrrole (Synlett 2014, 25, 423-427; ChemMedChem 2017, 12, 701-711), indole (Eur. J. Med. Chem. 2015, 93, 9-15; Biorg. Med. Chem. 2017, 25, 1122-1131) and pyrazole (Synlett 2014, 25, 2868-2872) afforded dipyrromethanes, bis(indolyl)methanes and bis(pyrazol-1-yl)methanes, respectively, via two consecutive hetero-Diels-Alder reactions (or conjugated additions) of the in situ generated nitrosoalkenes and azoalkenes.
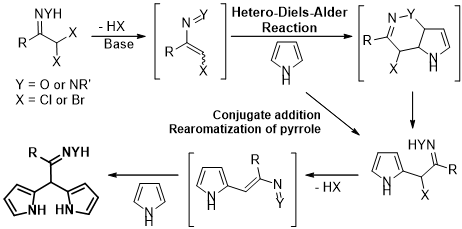
Having developed a route to meso-substituted dipyrromethanes we envisaged that the replacement of pyrrole by dipyrromethanes (DP) would lead to an approach to tetrapyrrolic compounds. In this context, a novel approach to trans-A2B-corroles has been developed. The synthetic strategy comprises the synthesis of bilanes from α,α-dihalo-oximes and dipyrromethanes followed by oxidative macrocyclization to give the target tetrapyrrolic macrocycles (J. Org. Chem. 2020, 85, 3328-3335; Phys. Chem. Chem. Phys. 2023, 25, 10263-10277). Recently, a synthetic route to trans-A2B-corroles, combining the macrocyclic core with a hydrazone moiety has also been developed (J. Med. Chem. 2024, 67, 21934-21951). The new macrocycles proved to be very efficient Photosensitizers for PDT of Lung Cancer.
Corroles from Nitrosoalkenes and Dipyrromethanes
The study of the photophysical properties of the novel hydrazone-functionalized trans-A2B-corroles combining the macrocyclic allowed the identification of photosensitizers with absorption within the phototherapeutic window and high singlet oxygen quantum yield. Relevant structure−photodynamic activity correlations were established by studying the new corroles-based photodynamic therapy (PDT) in human lung cancer cell lines (A549 and H1299). The methyl-hydrazone corroles were more active than phenylhydrazone corroles, with the N-Boc and N-Ts groups being key structural features to ensure high activity. The lead photosensitizers, with IC50 values below 100 nM and no cytotoxicity per se, were significantly more active than 5,10,15-triphenylcorrole, showing that the presence of the hydrazone functional group has a strong influence on PDT activity. Overall, the photodynamic activity of the novel trans-A2B-corroles make them very promising photosensitizers for use in PDT of lung cancer (J. Med. Chem. 2024, 67, 21934-21951).

Studies are underway to develop synthetic routes to other contracted porphyrins besides corroles, namely triphyrins and expanded porphyrins (hexaphyrins), as well as BODIPYs, by exploring nitroso and azoalkene chemistry. The focus is the development of compounds with the required features for their application as efficient therapeutic or luminescence imaging agents of lung cancer.
Spiro-b-Lactams as Novel Broad-Spectrum Host-Directed Antiviral
This is a project in the area of Medicinal Chemistry, anchored on fundamental organic chemistry. It started with a PhD project, carried out under Pinho e Melo’s supervision, focused on the development of new spiropenicillanates with potential biological activity. Collaborations were then established with Prof. Nuno Taveira (ISCSEM and iMed ULisboa, HIV Evolution, Epidemiology and Prevention Group) and Prof. Miguel Prudêncio (Research on Malaria, Instituto de Medicina Molecular, Lisbon). The study led to the discovery of new biologically active scaffolds, novel spiro-lactams with combined anti-HIV and anti-Plasmodium activities.
HIV infection and malaria remain serious public health problems which prevent economic and social progress in developing countries. The considerable geographic overlap between HIV and Plasmodium, mainly in sub-Saharan Africa, where coinfection (MHC) is common, contributes to the spread of both diseases. Therefore, new and better therapeutic drugs, effectively targeting both microorganisms, are urgently needed to treat and prevent these diseases, as current therapeutic options are limited and drug resistance is rising globally. Our research on spiro-beta-lactams led to the discovery of promising compounds with remarkable anti-HIV and anti-plasmodial properties [Pinho e Melo, Taveira et al. ACS Infectious Diseases 2021, 7, 421-434].
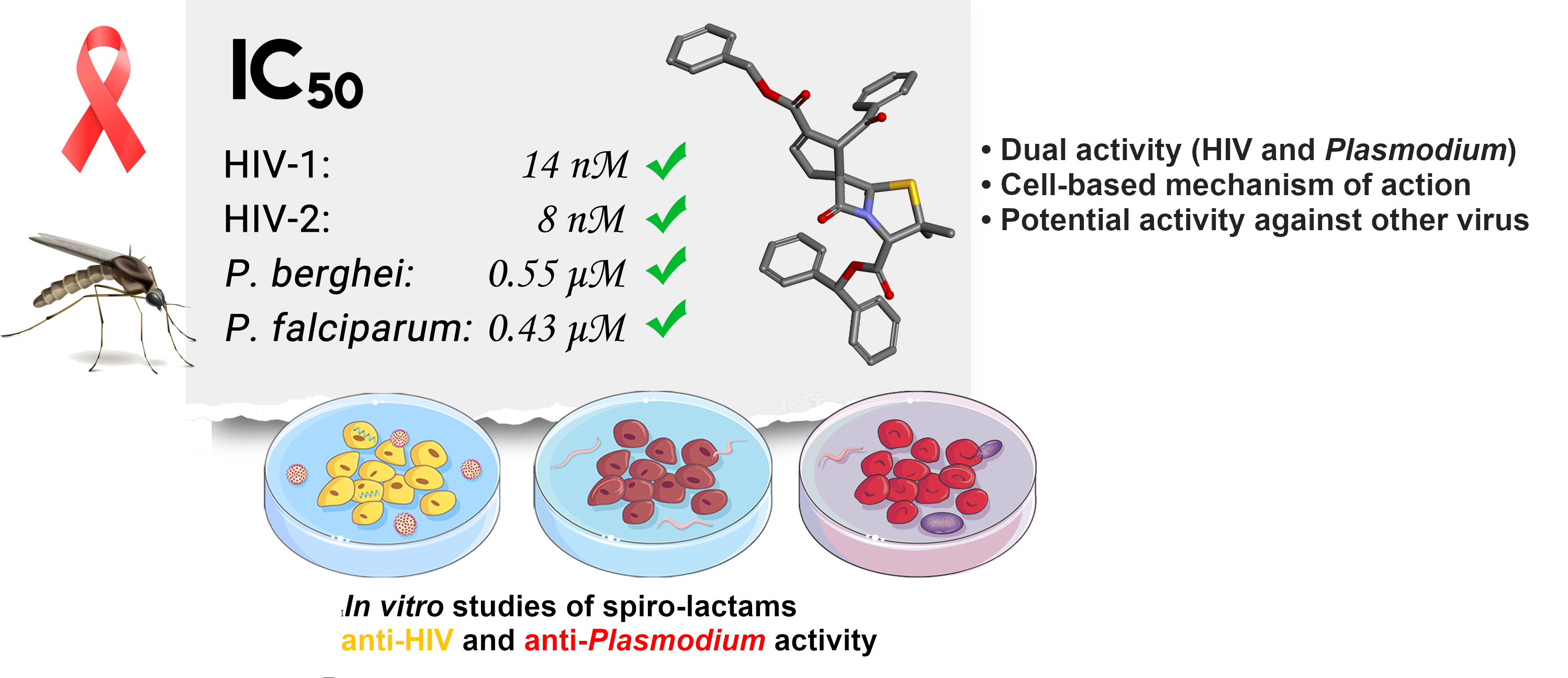
The design of new molecules based on the structural modulation of previously identified lead spiro-penicillanate (BSS-730A) was carried. The study provided relevant structure-activity relationship information leading to the discovery of new spirocyclopentenyl-β-lactams with remarkable nanomolar activity against HIV-1 also showing promising antiplasmodial activity, inhibiting both the hepatic and blood stages of Plasmodium infection. These results strengthen the potentiality of spiro-penicillanates as innovative and highly promising agents with dual activity against both HIV and Plasmodium [Curr. Top. Med. Chem. 2020, 20, 140-152; Eur. J. Med. Chem. 2021, 219, 113439; Front. Chem. 10:1017250 (2022)].
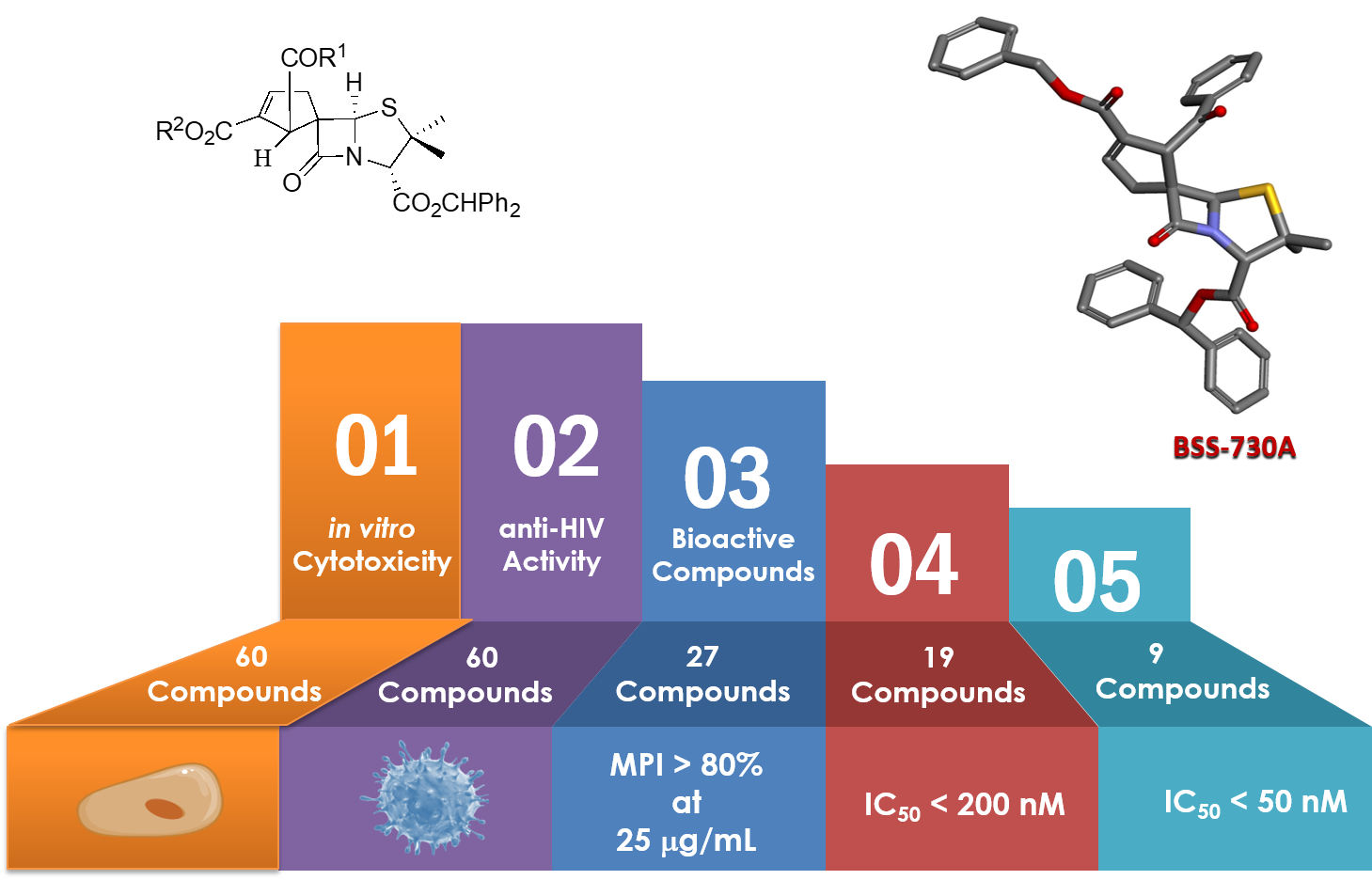
BSS-730A’s Derivatives with Anti-HIV Activity
Recent developments suggest a novel and complex mechanism of action. The new molecules act at the cellular level of the host, suggesting potential activity against other viruses, which has recently been confirmed. Indeed, the new spiro-penicillanates also showed remarkable activity against respiratory viruses. Research is now focusing on establishing the novel spiro-b-lactams as broad-spectrum host-directed antimicrobial agents, which may contribute to Increased preparedness against emerging viral threats and future pandemic situations.

Development of New P53 Activators
Following our publications on the study of 6,7-bis(hydroxymethyl)-1H,3H-pyrrolo[1,2-c]thiazoles as anticancer agents against breast cancer [Eur. J. Med. Chem. 2010, 45, 4676; Eur. J. Med. Chem. 2013, 60, 254; Eur. J. Med. Chem. 2014, 79, 273] a new collaboration has been established with, Prof. Lucília Saraiva, which led to the discovery of molecules with impressive properties as New Selective p53 Activators.
The State-of-the-Art anticancer drug development is currently focused on targeted therapies, which constitute the foundation of precision medicine. The tumor suppressor p53 is the most commonly inactivated protein in human cancers. The p53 protein functions as a sequence-specific transcription factor, initiating a cascade of cellular responses in order to prevent the replication of damaged DNA or the proliferation of genetically altered cells that can lead to tumor formation. Depending on the extension and severity of the stress stimulus and on the cell/tissue context, p53 may trigger a variety of cellular responses, including cell cycle arrest, DNA repair, senescence, apoptosis, and metabolic reprogramming. Impairment of the p53 pathway is a critical event in cancer. Therefore, reestablishing p53 activity has become one of the most appealing anticancer therapeutic strategies.
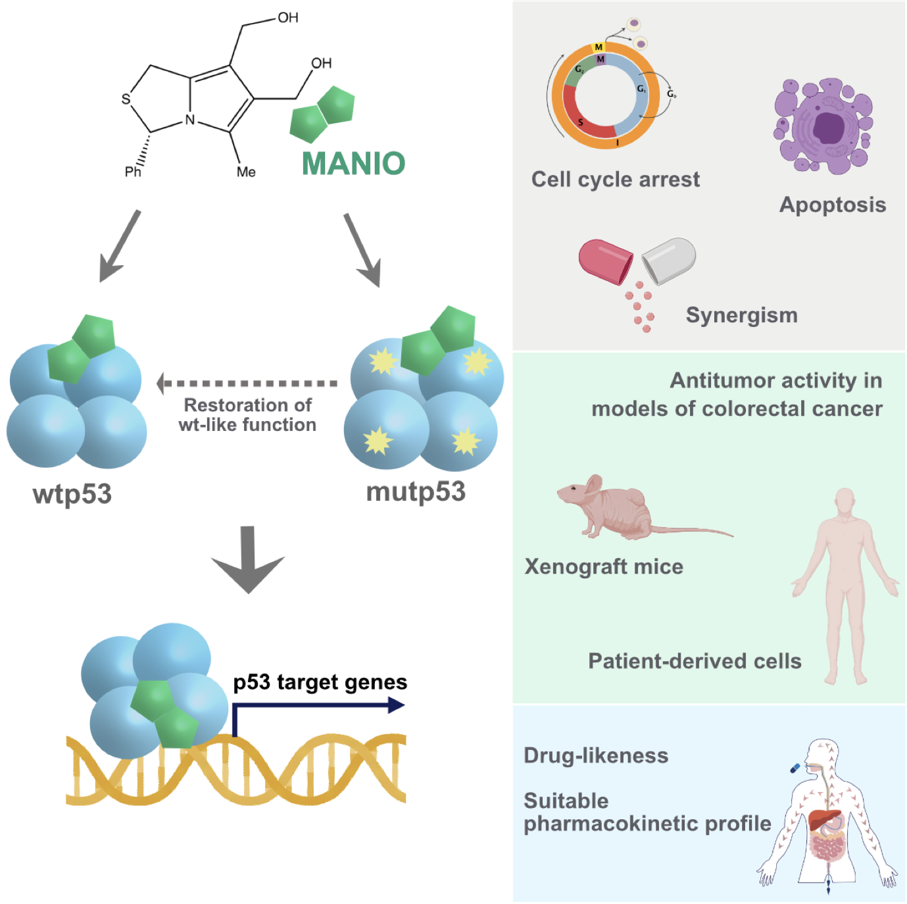
Recently, we have disclosed the p53-activating anticancer drug (3S)-6,7-bis(hydroxymethyl)-5-methyl-3-phenyl-1H,3H-pyrrolo[1,2-c]thiazole (MANIO) [Pinho e Melo, Lucília Saraiva et al. Cell Reports 2021, 35, 108982]. MANIO demonstrated a notable selectivity to the p53 pathway, activating wild-type (WT)p53 and restoring WT-like function to mutant (mut)p53 in human cancer cells. MANIO directly binds to the WT/mutp53 DNA-binding domain, enhancing the protein thermal stability, DNA-binding ability, and transcriptional activity. The high efficacy of MANIO as an anticancer agent toward cancers harboring WT/mutp53 was further demonstrated in patient-derived cells and xenograft mouse models of colorectal cancer (CRC), with no signs of undesirable side effects. MANIO synergizes with conventional chemotherapeutic drugs, and in vitro and in vivo studies predict its adequate drug-likeness and pharmacokinetic properties for a clinical candidate. As a single agent or in combination, MANIO will advance anticancer-targeted therapy, particularly benefiting colorectal cancer patients harboring distinct p53 status.
These remarkable results justify further studies on 6,7-bis(hydroxymethyl)-1H,3H-pyrrolo[1,2-c]thiazoles as new P53 Activators.
Valorization of Colophony from Pine Rosin

The RN21 Integrated Project, brings together the companies of the resin sector in Portugal, alongside partners from the production, industry, market, national scientific and technological system. The objective of RN21 is to promote Natural Resin as a biological and renewable raw product. The focus on natural and biobased products such as natural resin through innovative and transforming processes will contribute to a sustainable and carbon-neutral economy which will strengthen the national bioeconomy.
RN21 is financed by European funds allocated to Portugal by the Recovery and Resilience Plan (PRR), within the scope of the Recovery and Resilience Mechanism (MRR) of the European Union (EU) – (2021-2026).
The Organic Chemistry Group together with Rui Fausto’s research group participate in the RN21 with two projects carried out in collaboration with the company United Resins [Project RESFINAS and Project ResetFinas – PI: Teresa Pinho e Melo; Duration: 36 months (2022/2025)].
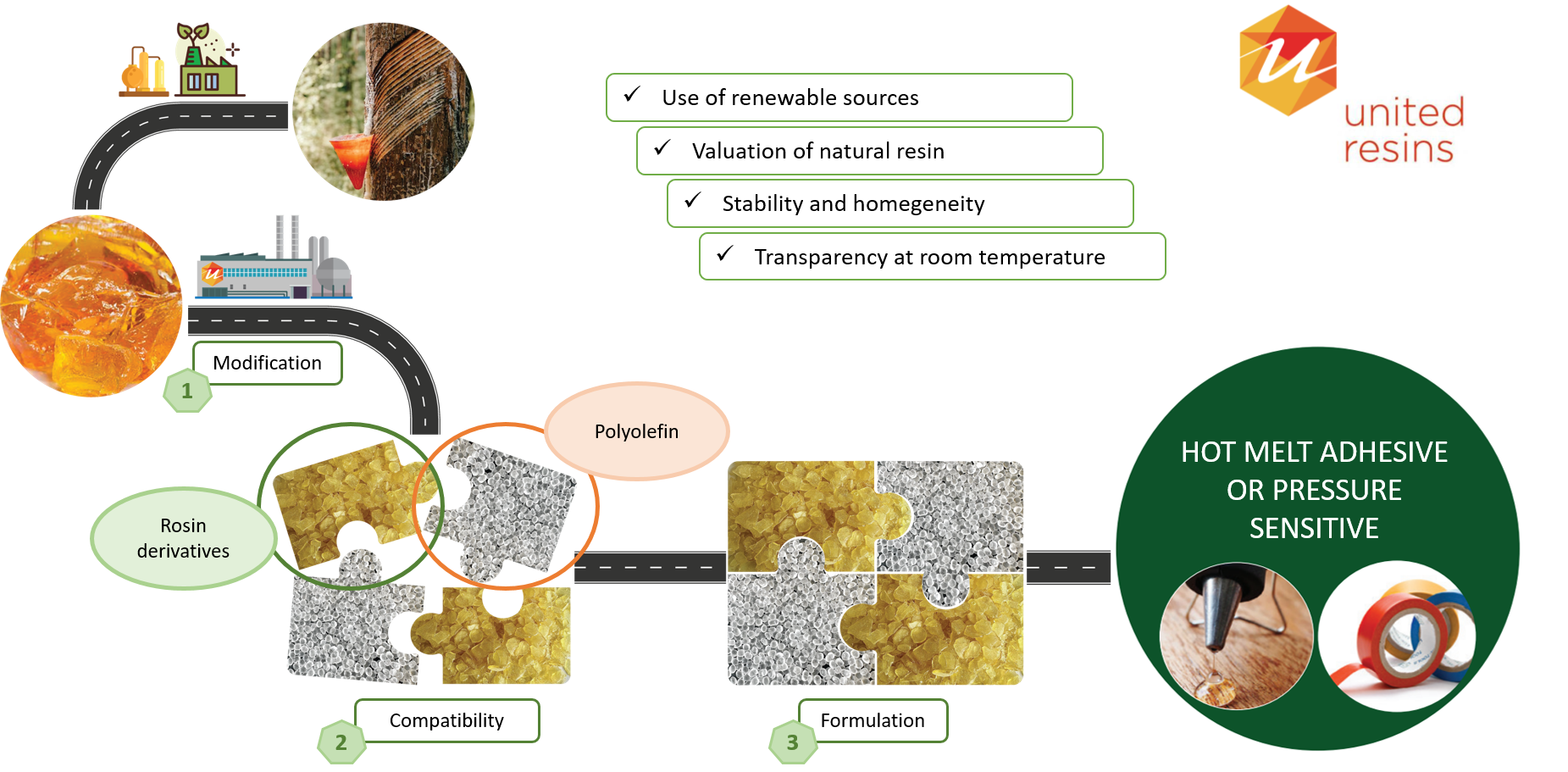
Adhesive formulations (Hot Melt or Pressure Sensitive Adhesives) typically include a polyolefin and a tackifying resin. Colophony resins are adhesive, but their use in formulations with polyolefins is limited by the high incompatibility of the two materials, mainly due to the low miscibility and the difference in polarity of the two components. The main objective of these projects is to make rosin compatible with polyolefins produced by catalysis with metallocenes, by structural modification of colophony.
Our ultimate goal on this research topic is the valorization of colophony as a biological and renewable raw product for the production of add- value products.
Heterogeneous and Asymmetric Catalysis
Elisa Serra and Dina Murtinho have been carrying out studies on Heterogeneous and Asymmetric Catalysis.
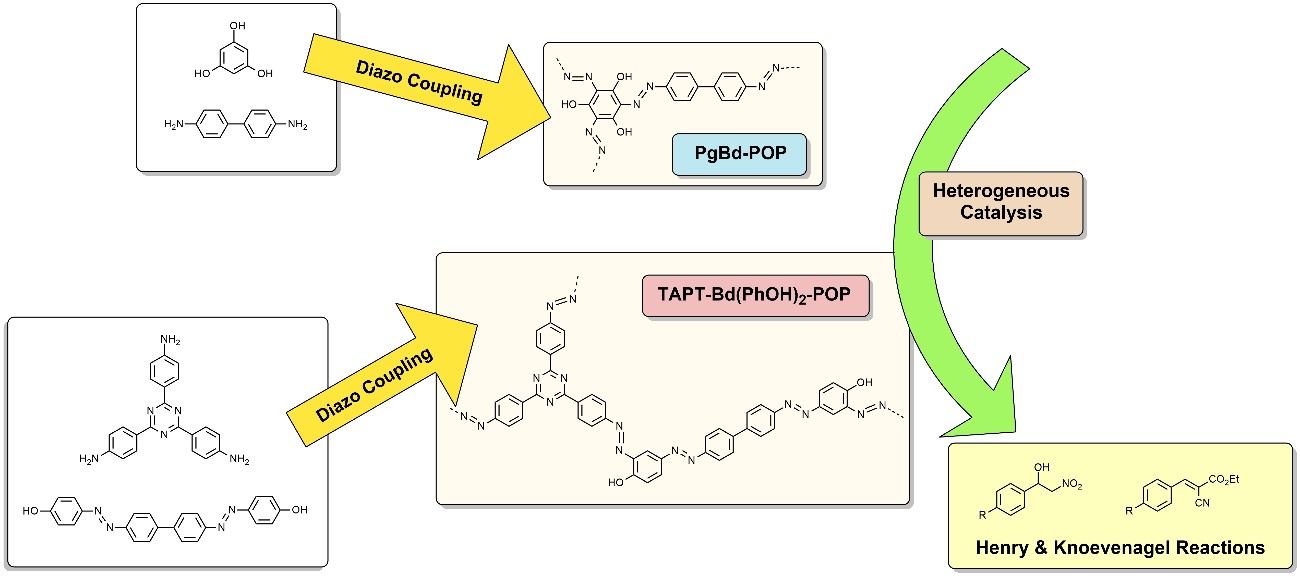
The synthesis and modification of porous organic polymers (POP), including covalent organic frameworks (COFs) are being carried out. These polymers are being used for heterogeneous catalysis, namely in Henry, Knoevenagel and multicomponent reactions. The chiral version of these reactions is also in study. The synthesized materials have also been used for the removal of pollutants. [Polymers 2022, 14(15), 3096; Microporous Mesoporous Mater, 355, 2023, 112561].
The synthesis and development of chiral ligands and organocatalysts using chiral natural compounds as starting materials to be used as catalysts in enantioselective alkylations, Henry, Michael and Strecker reactions, have also been studied.
[Appl Organomet Chem.2022;36:e6567; Appl Organomet Chem.2021;35:e6175]
The synthesis and modification of natural polymers and surfactants are also research topics. [Chemical Engineering Journal 455 (2023) 140882] [Journal of Molecular Liquids 342 (2021) 117389]
Green Chemistry
Marta Pineiro’s research group is dedicated to Green Chemistry and the development of sustainable synthetic procedures for the synthesis of heterocycles with unique photophysical properties and biological applications.
The goal of Green Chemistry, summarized in the 12 principles of Green Chemistry, is to reduce pollution at its sources by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents and products, looking at the solvent used in the process, exploring the potential use of catalysts, seeking the incorporation of renewable feedstocks, reducing energy utilization and identifying materials that do not persist or bioaccumulate.
The development of synthetic processes in line with the 12 principles of Green Chemistry implies the development of new technologies, such as microwave assisted synthesis or mechanochemistry, as well as the development of new synthetic strategies. These have been the two main research focuses of our group.
Microwave-assisted synthesis (Curr. Org. Synth 2014, 11, 89-109; Inorg Chim. Acta 2017, 455, 364-377; J. Porphyr. Phthalocyanines, 2019, 889-897); Dyes and Pigments 2020, 173, 107935) and mechanochemistry (ACS Omega 2020, 5, 19, 10868–10877; Green Chem. Lett. Rev, 2021, 14:2, 339-344) have proven to be powerful tools for increasing the sustainability of chemical processes, reducing or eliminating the need of solvent, allowing the use of greener solvents (such as water), decreasing the reaction time (energy input) required for the reaction and increasing the reaction yields.
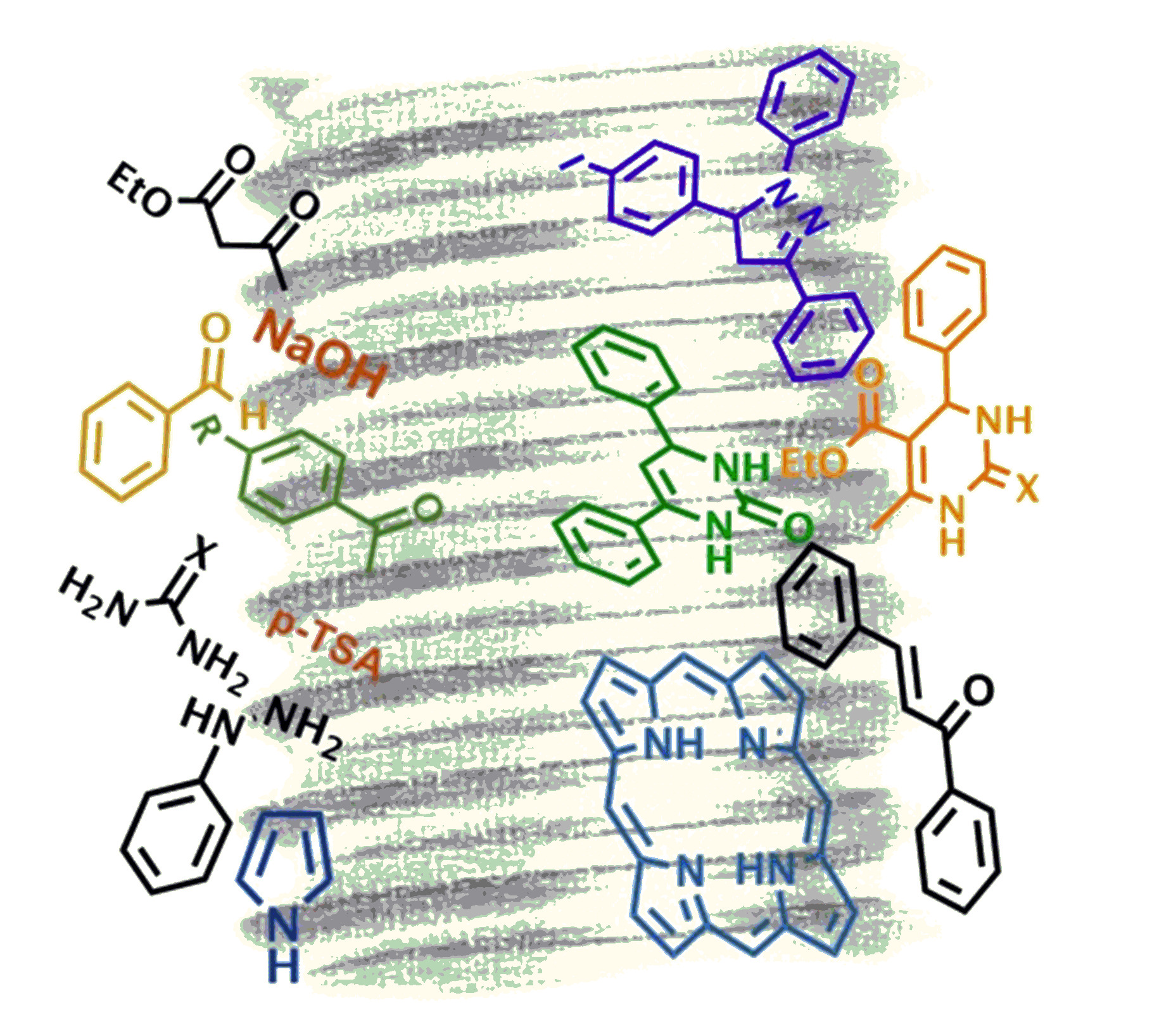
Multicomponent synthesis (Eur. J. Org. Chem. 2021, 113102; ACS Med. Chem. Lett. 2021, 12, 11, 1718–1725) and one-pot methodologies (Curr. Microw. Chem. 2014, 1, 119-134) have been explored for the synthesis and derivatization of heterocycles allowing to reduce the number of steps and reactions, and therefore waste, to obtain libraries of chemical compounds with high structural diversity.
The combination of new tools and new synthetic strategies to increase the overall sustainability of the synthetic processes is the ongoing project.
Sustainable Synthesis and Catalysis
Anthony Burke’s main interest is in drug discovery, with a particular focus on neurodegenerative disease and cancer targets. The group uses sustainable metal catalyzed approaches to access their core structure.
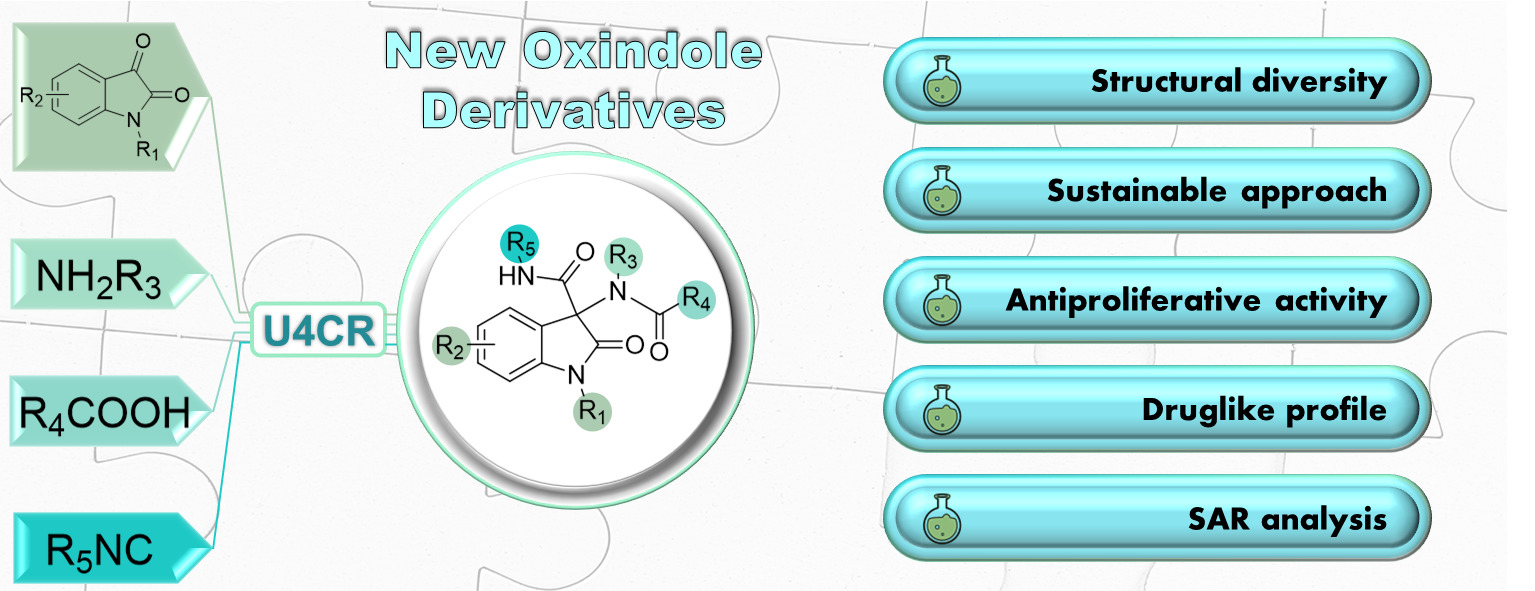
For example, a one-pot sequential Cu-catalyzed Chan-Lam/Pd-catalyzed carbonylation to give the anti-depressant Clozapine, 3-amino-3-aryl-oxindole butyrylcholinesterase (BuChE) inhibitors (Eur. J. Org. Chem. 2016, 806), choman-4-amine BuChE inhibitors and N-propargylchroman-4-amine monoamine-oxidase inhibitors (Bioorg. Med. Chem., 2022, 68, 116807), as well as key oxindole-triazole derivatives, that function as both BuChE inhibitors (Bioorganic Chem. 2020, 98, 103753) and anti-tumour and anti-lymphoma compounds (RSC Med. Chem. DOI: 10.1039/D2MD00044J.and EP3400938).
The group has also worked in the field of multi-component reactions for accessing drug-like molecules, using Bigenilli reactions (Synth.Comm. 2021, 51, 2954), as well as in collaboration with Marta Piñeiro (Chemistry Dept/CQC-University of Coimbra) and Carolina Marques (LAQV-University of Evora), Ugi-reactions (ACS Med. Chem. Lett. 2021, 12, 1718. Asian J. Org. Chem. 2021, 10, 1-23) and Petasis reactions (New J. Chem. 2021, 45, 14633, Eur. J. Org. Chem.2020, 3622).
The group is also interested in continuous-flow organocatalytic methods to access important pharmaceutical targets, which were developed at the University of Evora in collaboration with industry (Chiratecnics (www.chiratecnics.com) and the Swedish company Enginzyme (www.enginzyme.com)) and applied in enantioselective Michael additions (Amorim et al. Synlett, 2022, 33, 1756).

The group is also currently developing in collaboration with Luis Branco (New University of Lisbon) Jorge Morgado and Henrique Gomes (Institute of Telecommunications, IST-UL) on new opto-electronic organic molecules for applications in the electronics and superconductor industries (Chem. Comm. 2020, 14893). This has been recently financed with an FCT grant; ConChiMOL - New Structurally Contorted and Chiral Molecules for Optoeletronic Applications, 2022.01391.PTDC.