Shedding light on intramolecular vibrational energy redistribution.
 The detailed understanding of photochemical processes at the molecular level requires the investigation of both the excitations as well as the relaxation mechanisms. One direction of our research is dedicated to studies of the processes of intramolecular vibrational energy redistribution (IVR), i.e. how the excess energy of vibrationally excited molecules can be dissipated via intramolecular relaxation pathways.
The detailed understanding of photochemical processes at the molecular level requires the investigation of both the excitations as well as the relaxation mechanisms. One direction of our research is dedicated to studies of the processes of intramolecular vibrational energy redistribution (IVR), i.e. how the excess energy of vibrationally excited molecules can be dissipated via intramolecular relaxation pathways.
The monomers of conformationally flexible compounds are captured in cryogenic (typically at 4-15 K) inert matrices, and their isomerizations, induced by selective vibrational excitations, are probed. The detailed characterization of the reactants and products is carried out by infrared spectroscopy. Interpretation of the experiments is aided by fully anharmonic calculations of the fundamental modes, overtones, and combinations up to two quanta, including their infrared intensities.
In this way we have been gaining insight into the IVR processes and contributing to development of vibrationally controlled molecular switches.
- “Conformational Switching in Pyruvic Acid Isolated in Ar and N2 Matrixes: Spectroscopic Analysis, Anharmonic Simulation, and Tunneling”, I. Reva, C. M. Nunes, M. Biczysko and R. Fausto, J. Phys. Chem. A, 119 (2015) 2614.
- “Conformational Switching by Vibrational Excitation of a Remote NH Bond”, A. J. Lopes Jesus, I. Reva, C. Araujo-Andrade and R. Fausto, J. Am. Chem. Soc. 137 (2015) 14240. (Front cover, JACS).
- “Near-Infrared In Situ Generation of the Higher-Energy Trans Conformer of Tribromoacetic Acid: Observation of a Large-Scale Matrix-Site Changing Mediated by Conformational Conversion”, R. F. G. Apóstolo, G. Bazsó, G. O. Ildiz, G. Tarczay and R. Fausto, J. Chem. Phys. 148 (2018) 044303.
- “Investigation of Long-Range Intramolecular Vibrational Energy Redistribution by NIR Irradiation Induced Conformational Transformation of E-Glutaconic Acid”, B Kovács, N. Kus, G. Tarczay and R. Fausto, J. Phys. Chem. A. 121 (2017) 3392.
Structure and photoinduced transformation of electronically controlled molecular switches.
 Parent azobenzene (AB) and 2,2'-substituted AB (R=OH, CH3) were isolated in argon and xenon matrices and their molecular structures and photochemical transformations were characterized by infrared spectroscopy and theoretical calculations. All these compounds can adopt the E and Z isomeric forms around the central CNNC moiety. For AB, both E → Z and Z → E isomerizations were observed upon irradiations with UV and visible light (contrary to what was observed in an Ar matrix). The studies of isomerization between the two 2,2'‑substituted AB derivatives provided a direct experimental observation of an E → E isomerization. The latter type of photoisomerization suggests the occurrence of a small‑amplitude pedal motion for azobenzene-type molecules in condensed phase environments.
Parent azobenzene (AB) and 2,2'-substituted AB (R=OH, CH3) were isolated in argon and xenon matrices and their molecular structures and photochemical transformations were characterized by infrared spectroscopy and theoretical calculations. All these compounds can adopt the E and Z isomeric forms around the central CNNC moiety. For AB, both E → Z and Z → E isomerizations were observed upon irradiations with UV and visible light (contrary to what was observed in an Ar matrix). The studies of isomerization between the two 2,2'‑substituted AB derivatives provided a direct experimental observation of an E → E isomerization. The latter type of photoisomerization suggests the occurrence of a small‑amplitude pedal motion for azobenzene-type molecules in condensed phase environments.
- “Controlled Light-driven Switching in 2-Thiobenzimidazole”, E. M. Brás and R. Fausto, J. Photochem. Photobiol. A: Chemistry 357 (2018) 185.
- “Photoisomerization of Azobenzenes Isolated in Cryogenic Matrices”, L. Duarte, L. Khriachtchev, R. Fausto and I. Reva, Phys. Chem. Chem. Phys. 18 (2016) 16802.
- “Structural and Spectroscopic Characterization of E- and Z- Isomers of Azobenzene”, L. Duarte, I. Reva and R. Fausto, Phys. Chem. Chem. Phys. 16 (2014) 16919 (Inside Front cover, PCCP).
- "Tautomers and UV-Induced Photoisomerization of a Strongly Intramolecularly H-Bonded Aromatic Azo-Dye: 1-(Cyclopropyl)diazo-2-naphthol", L. Duarte, B.M. Giuliano, I. Reva, and R. Fausto, J. Phys. Chem. A 117 (2013) 10671.
Mechanistic insights on the generation and photochemistry of reactive species.
 Generation followed by stabilization in cryogenic matrices of reactive species like radicals and many other usually short-living reaction intermediates has been achieved, alowing their detailed structural, spectroscopic and photochemical characterization, and the investigation of their reactivity. Following our work on the photogeneration and photochemistry of the phenoxy radical, we shed light on the photochemistry of its sulphur analogue. Also, our work on the photochemical reactivity of tetrazoles explored strategies to generate a substantial number of rare, highly-reactive molecules, which were reviewed in a publication in JPP C – Photochem. Reviews.
Generation followed by stabilization in cryogenic matrices of reactive species like radicals and many other usually short-living reaction intermediates has been achieved, alowing their detailed structural, spectroscopic and photochemical characterization, and the investigation of their reactivity. Following our work on the photogeneration and photochemistry of the phenoxy radical, we shed light on the photochemistry of its sulphur analogue. Also, our work on the photochemical reactivity of tetrazoles explored strategies to generate a substantial number of rare, highly-reactive molecules, which were reviewed in a publication in JPP C – Photochem. Reviews.
- “Photogeneration of the Thione Forms of Thiophenol”, I. Reva, M. J. Nowak, L. Lapinski and R. Fausto, Phys. Chem. Chem. Phys. 17 (2015) 4775. (Inside front cover, PCCP)
- “Genesis of Rare Molecules Using Light-Induced Reactions of Matrix-Isolated Tetrazoles”, L.M.T. Frija, M.L.S. Cristiano, A. Gómez-Zavaglia, I. Reva and R. Fausto, J. Photochem. Photobiol. C: Photochem. Reviews, 18 (2014) 71.
- “Infrared Spectra and UV-Tunable Laser Induced Photochemistry of Matrix-Isolated Phenol and Phenol-d5”, B.M. Giuliano, I.D. Reva, L. Lapinski and R. Fausto, J. Chem. Phys. 136 (2012) 024505.
Experimental observation of bond-shift isomers.
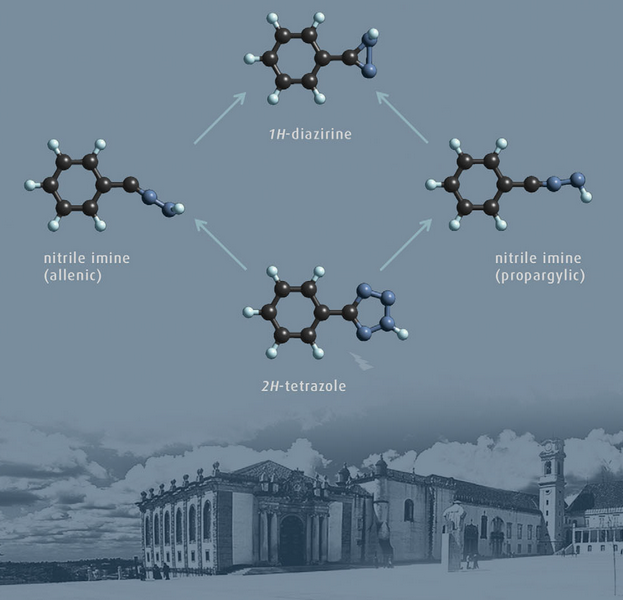 Bond-shift isomers have been theoretically postulated for many years, but escaped to experimental observation. We were able to produce and observed experimentally bond-shift isomers of phenylnitrile imine, the allenic and propargylic forms, and study their reactivity in details.
Bond-shift isomers have been theoretically postulated for many years, but escaped to experimental observation. We were able to produce and observed experimentally bond-shift isomers of phenylnitrile imine, the allenic and propargylic forms, and study their reactivity in details.
- “Bond-Shift Isomers: The Co-existence of Allenic and Propargylic Phenylnitrile Imines”, C. M. Nunes, I. Reva, R. Fausto, D. Bégué and C. Wentrup, Chem. Comm. 51 (2015) 14712. (Back cover, Chem. Comm.)
Production of small aggregates and energetic crystals made by rare conformers.
Preparation and characterization of high-energy conformers, dimers and complexes of these species has been achieved by controled conformational changes induced by narrow band selective excitation of low-energy common conformers isolated in cryogenic matrices, followed by thermal mobilization under temperature controled conditions. Procedures were developed for subsequent preparation of high-energy crystals based on these high-energy rare conformers.
Selected Recent References:
- “Formic Acid Dimers in a Nitrogen Matrix”, S. Lopes, R. Fausto and L. Khriachtchev, J. Chem. Phys. 148 (2018) 034301.
- “Acetic Acid - Water Complex: The first Observation of Structures Containing the Higher -Energy Acetic Acid Conformer”, S. Lopes, R. Fausto and L. Khriachtchev, J. Chem. Phys. 144 (2016) 084308.
- “The Birth of High-Energy Crystals from Isolated Molecules – The Unsuspected Parenthood of Rare Conformers”, Project PTDC/QEQ-QFI/3284/2014. (P. I. – Rui Fausto).
Development of “Vibrational Antennas” to induce structural changes at distance.
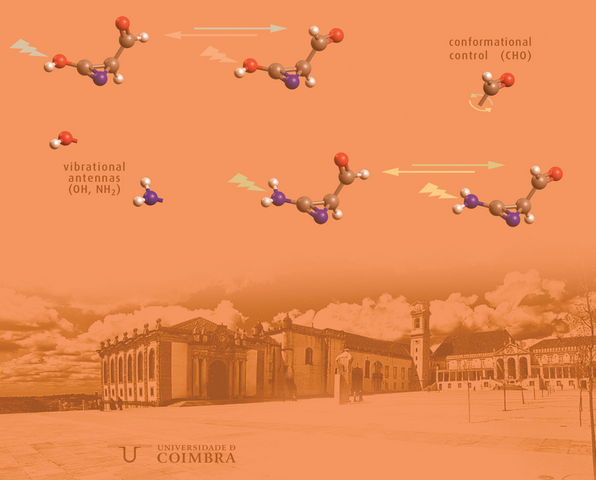 We demonstrated that intramolecular vibrational energy relaxation can occur at long distance, providing a mechanism for promoting highly selective structural transformations, most of the times reversible, in molecular fragments separated by several chemical bonds from the vibrationally excited group (antenna). These results open a gate for using Near-IR laser light excitation to selectively promote conformational isomerizations and also bond breaking/bond forming processes.
We demonstrated that intramolecular vibrational energy relaxation can occur at long distance, providing a mechanism for promoting highly selective structural transformations, most of the times reversible, in molecular fragments separated by several chemical bonds from the vibrationally excited group (antenna). These results open a gate for using Near-IR laser light excitation to selectively promote conformational isomerizations and also bond breaking/bond forming processes.
- “Bond-Breaking/Bond-Forming Reactions by Vibrational Excitation: Infrared-Induced Bidirectional Tautomerization of Matrix-Isolated Thiotropolone”, C. M. Nunes, Nelson A. M. Pereira, Igor Reva, Patrícia S. M. Amado, Maria L. S. Cristiano and Rui Fausto, J. Phys. Chem. Lett. 11 (2020) 8034
- “Conformational Control over an Aldehyde Fragment by Selective Vibrational Excitation of Interchangeable Remote Antennas”, A. J. Lopes Jesus, C. M. Nunes, R. Fausto and I. Reva, Chem. Comm. 54 (2018) 4778. (Inside Back cover, Chem. Comm.)
- “UV-Induced Transformatins in Matrix-Isolated 6-Methoxyindole”, A. J. Lopes Jesus, I. Reva and R. Fausto, J. Photochem. Photobiol. A: Chemistry 336 (2017) 123.
- “Conformational Switching by Vibrational Excitation of a Remote NH Bond”, A. J. Lopes Jesus, I. Reva, C. Araujo-Andrade and R. Fausto, J. Am. Chem. Soc. 137 (2015) 14240. (front cover, JACS).
Exploring the chemistry of nitrenes and tunneling reactions:
We have been investigating nitrenes reactive intermediates (open‑shell R‒N species) and their slippery potential energy surface using a combination of a cryogenic matrix environment and a tunable narrowband radiation source to selectively produce and induce photochemistry of the different species resulting from suitable initial nitrene precursors. In this way, we have unraveled new gateways to nitrenes, characterized new challenging species and discovered fascinating cases of tunneling reactions. Direct spectroscopic observations of heavy-atom tunneling reactions and competitive tunneling events have put our research group in the forefront of this research filed.
Selected Recent References:
- “Competitive Nitrogen vs Carbon Tunneling”, C. M. Nunes, I. Reva, R. Fausto, A. K. Eckhardt and P. R. Schreiner, J. Am. Chem. Soc. 141 (2019) 14340.
- “Photochemistry of 2-Formylphenylnitrene: A Doorway to Heavy-Atom Tunneling of a Benzazirine to a Cyclic Ketenimine”, C. M. Nunes, I. Reva, S. Kozuch, R. J. McMahon and R. Fausto , J. Am. Chem. Soc. 139 (2017) 17649.
- “Evidence of a Nitrene Tunneling Reaction: Spontaneous Rearrangement of 2-Formyl Phenylnitrene to an Imino Ketene in Low-Temperature Matrices”, C. M. Nunes, S. N. Knezz, I. Reva, R. Fausto and R. J. McMahon, J. Am. Chem. Soc. 138 (2016) 15287.